Solved overall reaction: 2NO(g)+O2(g)→2NO2(g) Step
4.8 (586) · € 35.00 · Auf Lager

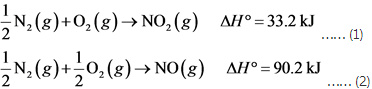
Calculate the enthalpy of the reaction 2NO(g)+O2(g)→2NO2(g) - Home Work Help - Learn CBSE Forum

Kinetics. - ppt download

The reaction 2NO + Br_{2} rightarrow 2NOBr follows the mechanism:(1) NO + Br_{2} overset{Fast}{rightleftharpoons} NOBr_2(2) NOBr_2 + NO xrightarrow{Slow} 2NOBrWhich of the following is/are true regarding this?The order of the reaction with
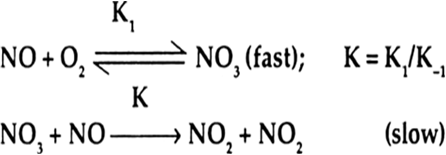
Nitric oxide, NO, reacts with oxygen to produce nitrogen dioxide
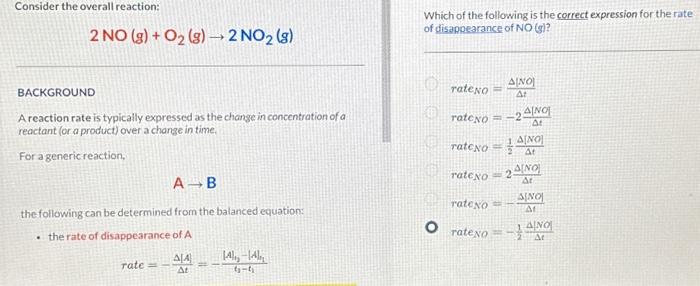
Solved Consider the overall reaction: 2NO(g)+O2( g)→2NO2( g)
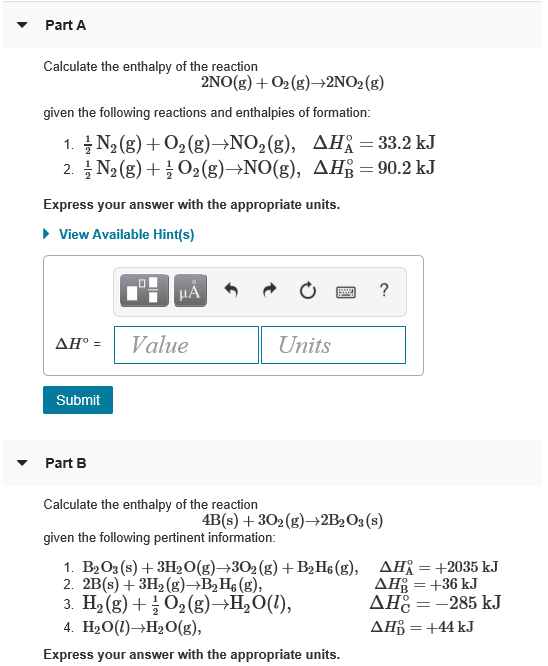
Solved Part A Calculate the enthalpy of the reaction 2NO(g)
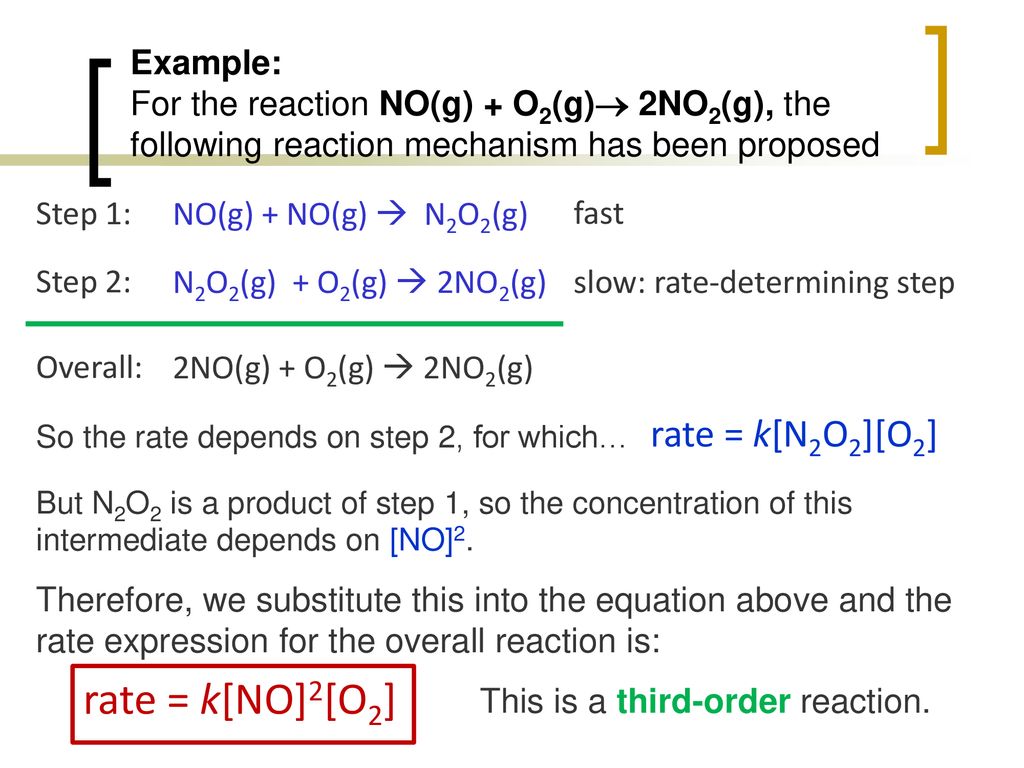
Part 3: Reaction Mechanisms - ppt download
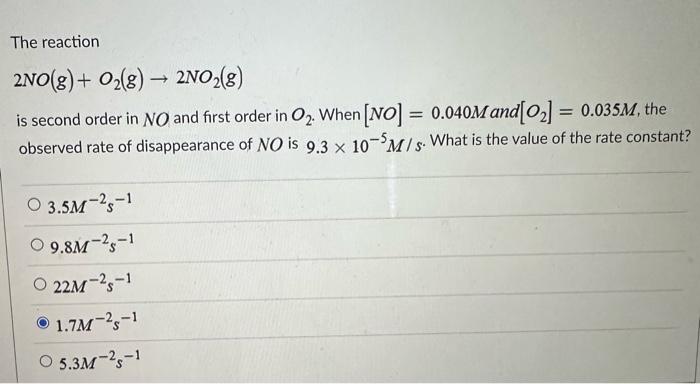
Solved The reaction 2NO(g)+O2(g)→2NO2(g) is second order in

consider the following reaction 2no(g)+o2(g)→2no2(g). calculate ∆g° at 298k and predict weather the reaction

For the reaction mechanism given in Problem 18-12, classify
![SOLVED: 2NO(g) + O2(g) -> 2NO2(g) For the above reaction, the following data were collected for the rate of disappearance of NO in the reaction: Initial Rate Experiment, [NO] (M)](https://cdn.numerade.com/ask_images/f3dbb5d2b4004b2b89a4c8cddfc9079c.jpg)
SOLVED: 2NO(g) + O2(g) -> 2NO2(g) For the above reaction, the following data were collected for the rate of disappearance of NO in the reaction: Initial Rate Experiment, [NO] (M)
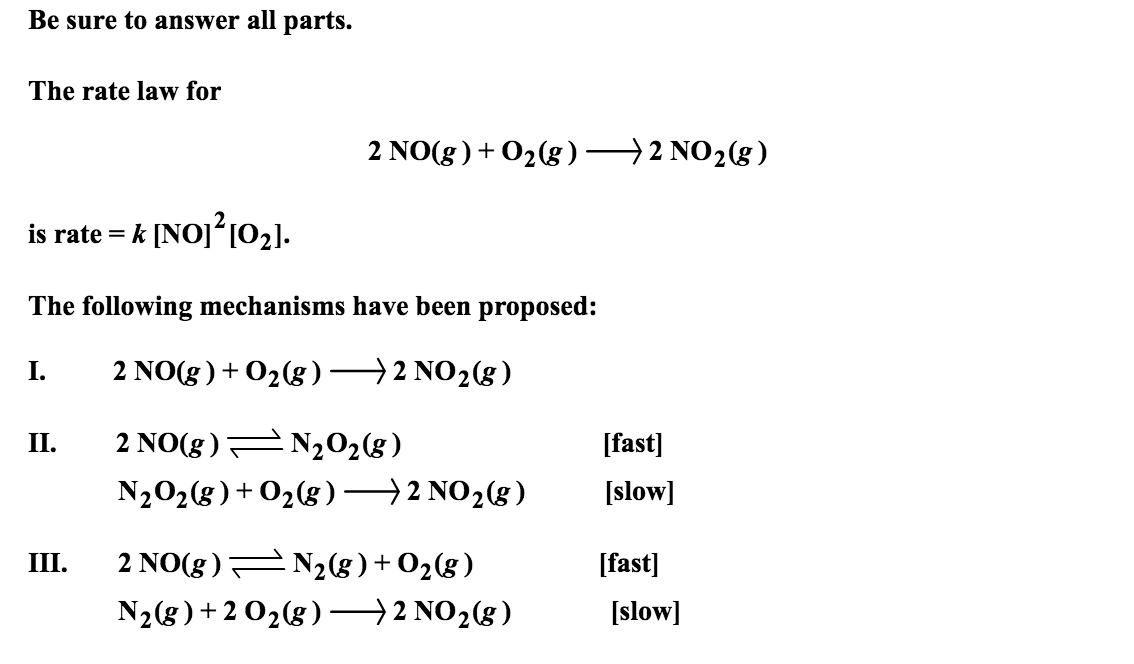
Solved Be sure to answer all parts. The rate law for 2NO(g )

Solved) - The Reaction 2NO2 ? 2NO + O2 Obeys The Rate Law: Rate = 1.4 X (1 Answer)
For the reaction, N2(g) + O2(g) ⇋ 2NO(g), the equilibrium constant is K1. - Sarthaks eConnect