FDA Outlines Generic Safety and Efficacy - Policy and Medicine
4.7 (285) · € 25.50 · Auf Lager
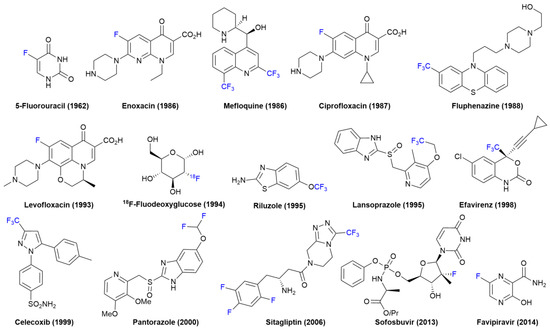
IJMS, Free Full-Text

3D printing for personalised medicines: implications for policy and practice - ScienceDirect
Regenerative Medicine: Therapeutic Applications, Challenges, and Policy Options
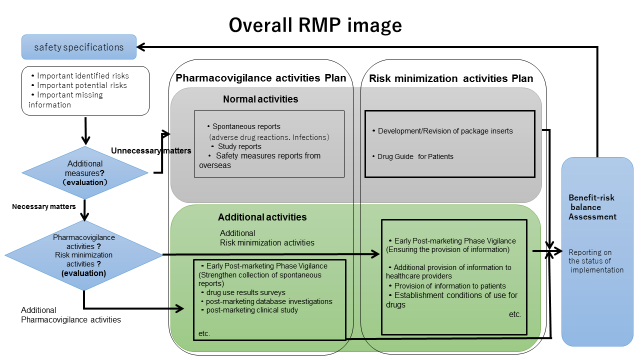
Risk Management Plan (RMP) Pharmaceuticals and Medical Devices Agency
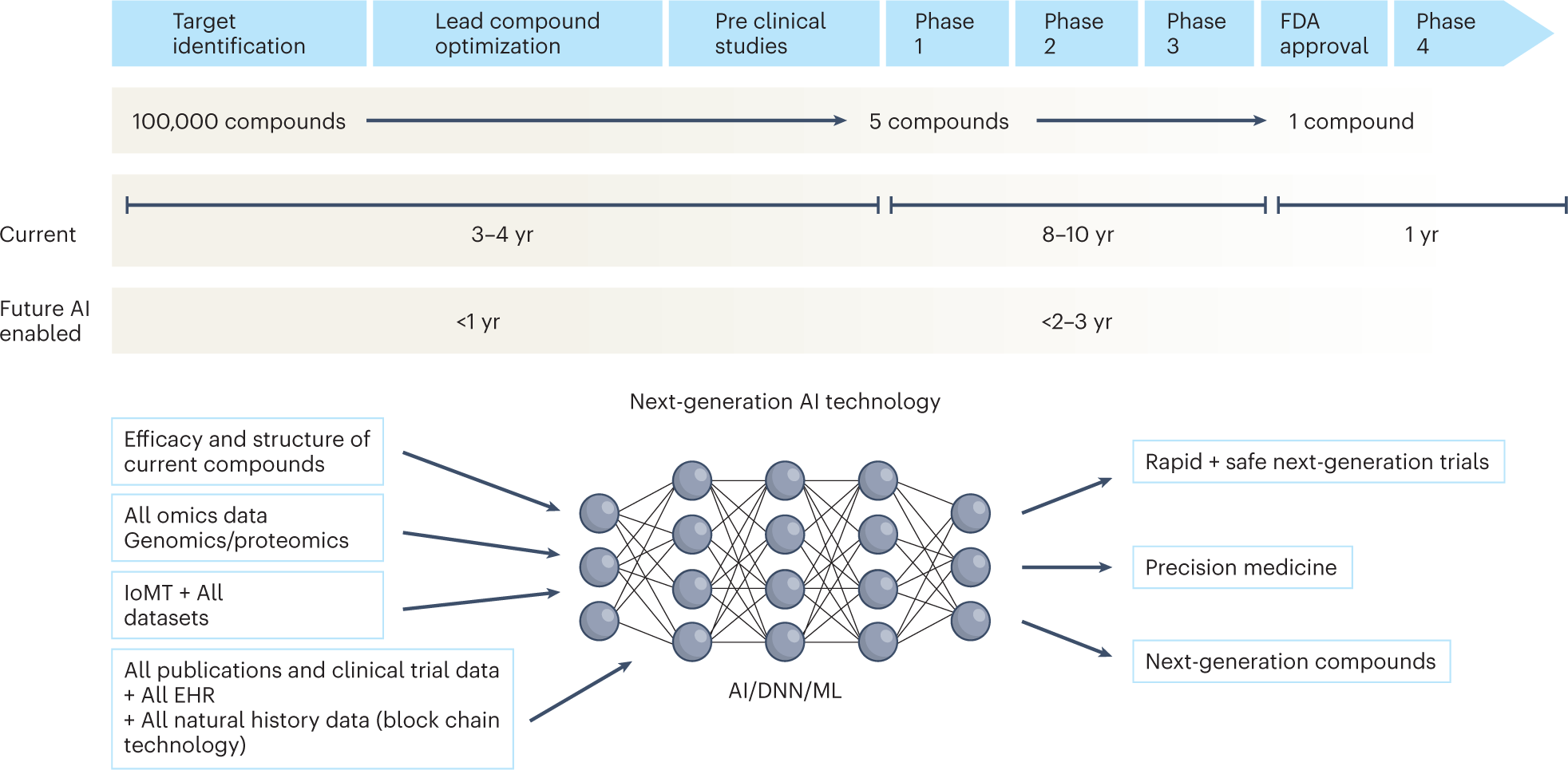
The next generation of evidence-based medicine

FDA 21 CFR Part 820 Compliance for Medical Device Companies
FDA Outlines Generic Safety and Efficacy - Policy and Medicine

Home - Food and Drug Administration

Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 - BioProcess InternationalBioProcess International
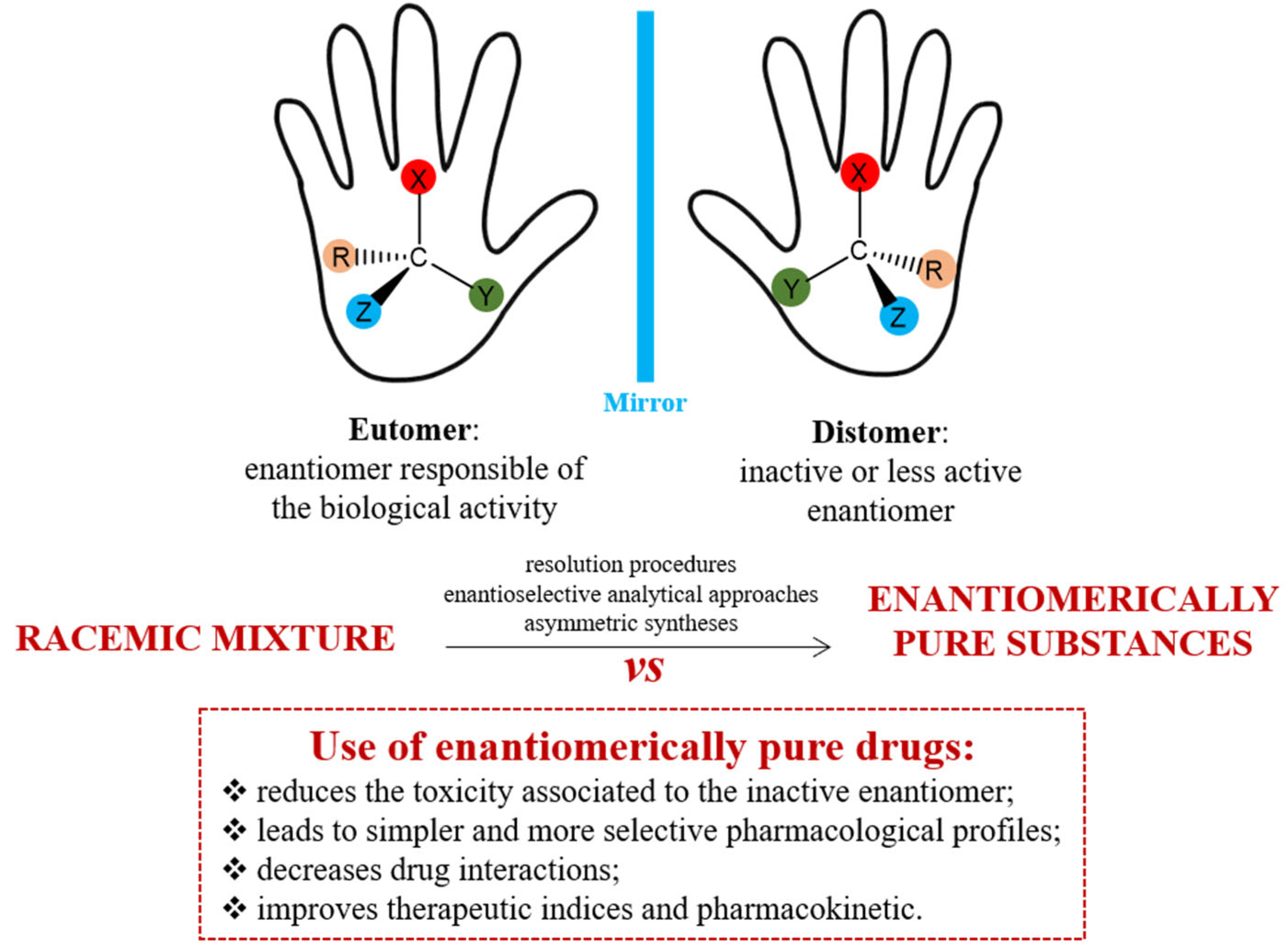
Applied Sciences, Free Full-Text

How FDA Approves Drugs and Regulates Their Safety and Effectiveness






:max_bytes(150000):strip_icc()/GettyImages-185235418-5b3e1cb646e0fb005b7c65e3.jpg)
