FDA: Global Pharma's eye drops contaminated with “filth” while
4.6 (153) · € 23.00 · Auf Lager
The US Food and Drug Administration (FDA) lambasted India-based Global Pharma Healthcare in a 14 November warning letter for manufacturing eye drops that have been linked to an outbreak of bacterial infections resulting in consumer injury, blindness, and at least three deaths. In a related enforcement action, FDA also rebuked for making unapproved drug claims.@

Three people die after using contaminated eyedrops bought at Walmart, CVS and Target: Eight lose vision and four have eyeballs surgically removed, Page 5

Drug GMP Warning Letter Issued Without Facility Inspection - Redica Systems
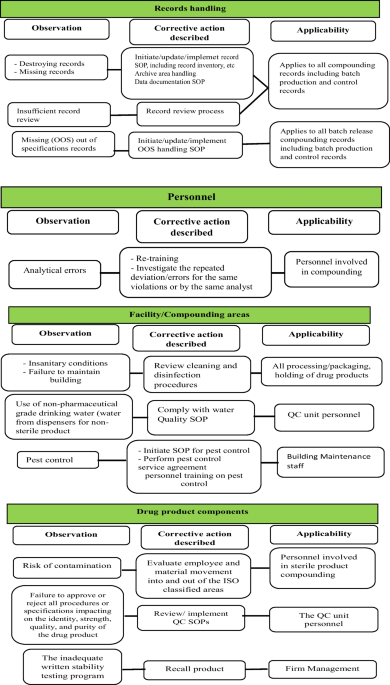
Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022
Pharma News: Pfizer Acquires Cancer Drug Company Seagen For 43 Billion US Dollars In Anticipation Of The Rise In Turbo Cancers! - Thailand Medical News
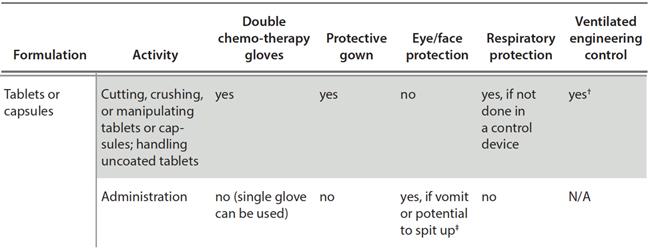
Road to USP 800 Compliance Archives - Visante
Arthritis Supplements Reviewed by

Untangling Antimicrobial Resistance (AMR) - The Legacy of an unhealthy development model by SocietyForInternationalDevelopment - Issuu

BEYC #673 - Bored Eye Yawn Club

PDF) Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022
European drug regulators abruptly reverse course on boosters

Silke Schumacher-Ben Hassine on LinkedIn: FDA: Global Pharma's eye drops contaminated with “filth” while made…
Another death, more cases of vision loss linked to contaminated eye drops, CDC reports, Health

Dollar Tree Gets FDA Warning For 'Potentially Unsafe Drugs







