Is CH3OH (Methanol) Soluble or Insoluble in Water?
5 (380) · € 31.50 · Auf Lager


Explain why Methanol (CH3OH) is soluble in water but Benzene (C6H6
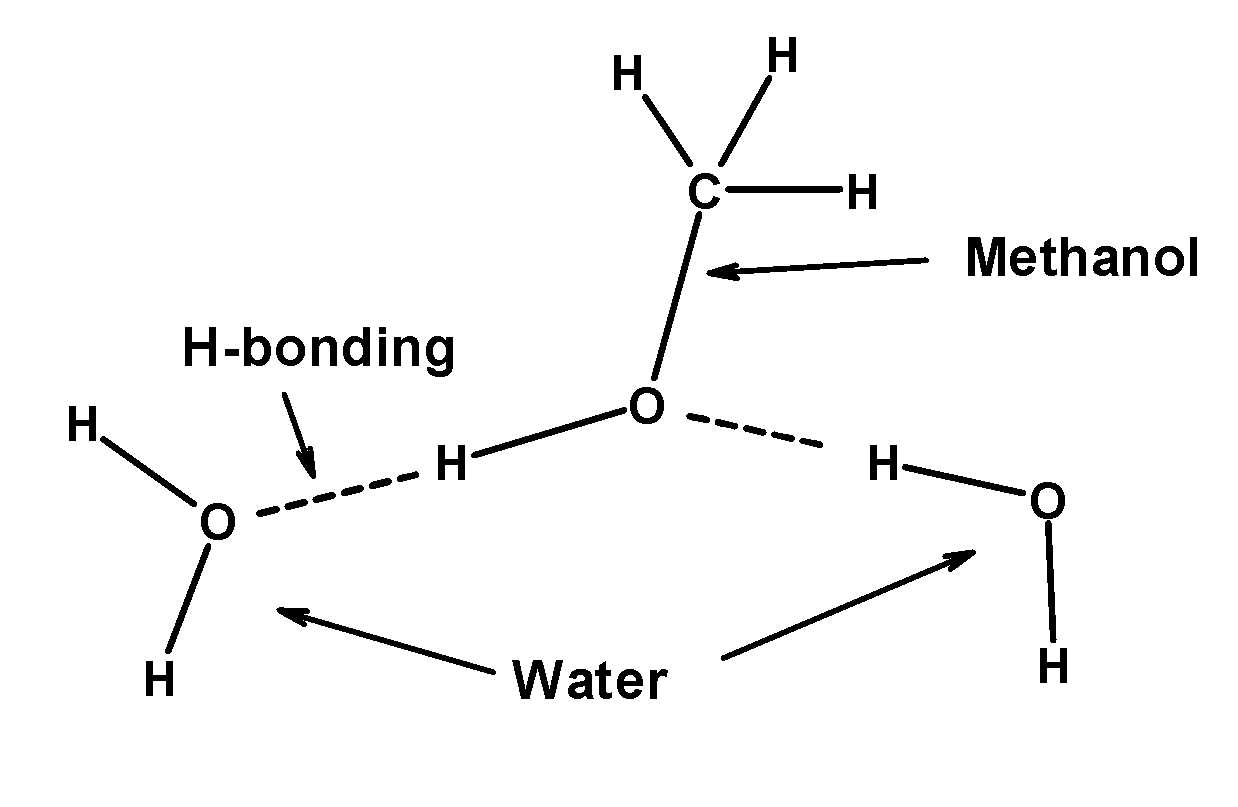
Though covalent in nature, methanol is soluble in water, why?A

CH3OH + H2O (Methanol + Water)
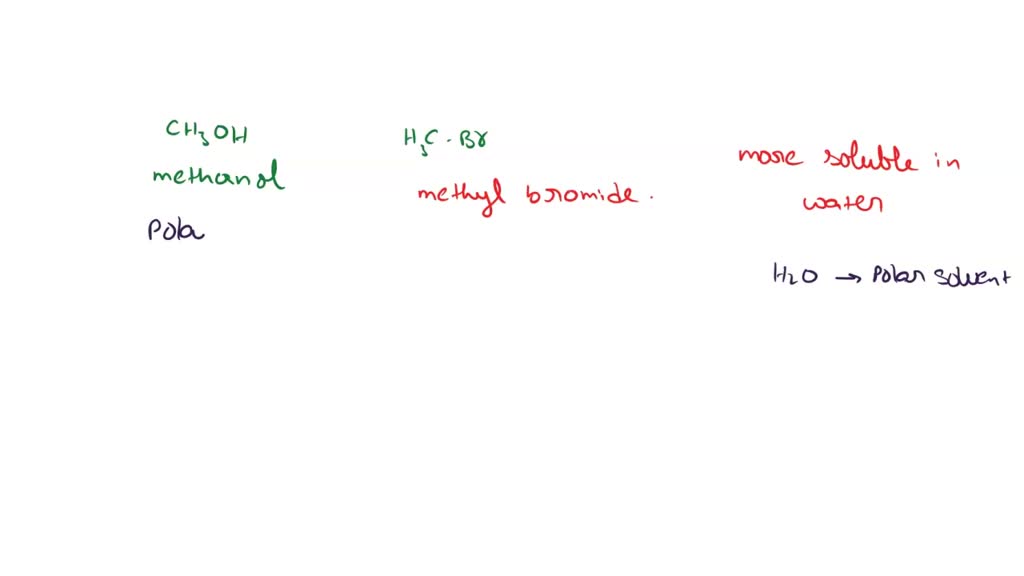
SOLVED: Which of the following is more soluble in water, methanol

Co-Nonsolvency Effect in Solutions of Poly(methyl methacrylate)-b
Solved B. Solutes and Solvents 1. Solubility SOLVENT water

Answered: 9. Which substance is most soluble in…
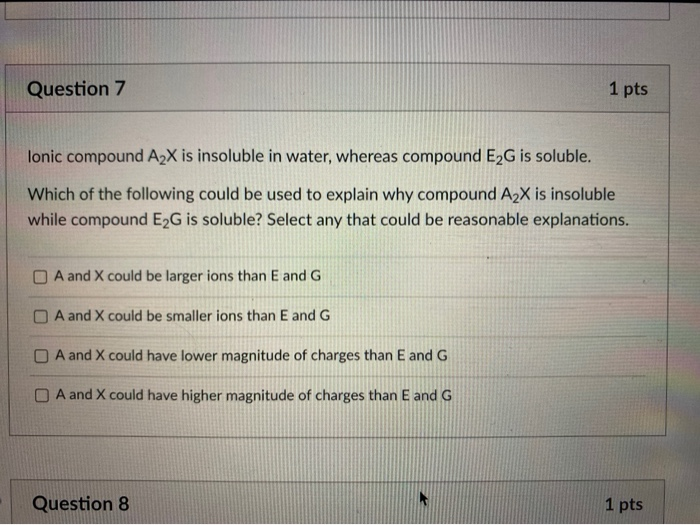
Solved What is wrong with this picture, showing CH3OH before

D17.4 Solutions and Solubility – Chem 109 Fall 2023

SOLVED: 10.110 Why is methanol (CH3OH) miscible with water

Which is more polar: H2O or CH3OH? - Quora

SOLVED: When methanol, CH3OH, is dissolved in water, a

Why is methanol soluble in water? - Quora